Sanochemia Pharmazeutika
Together for a
brighter life
PROVEN EUROPEAN EXPERTISE
Specialty active ingredients and pharmaceutical niche products
We are a high-performance pharmaceutical niche supplier in the heart of Europe. Our strength lies in the development and GMP-compliant production of active pharmaceutical ingredients (APIs) as well as drug products (FDF). In Austria, we develop and produce sterile injectables and small molecule APIs for our own use as well as CDMO for third parties. Our imaging agents for CT and MR are used for diagnostic imaging by physicians and hospitals in more than 50 countries worldwide.
For more than 130 years, the company has existed at the site in Austria and for more than 30 years we have been operating under the current name Sanochemia Pharmazeutika. We are valued by partners and customers as a reliable producer of high-quality active ingredients and pharmaceuticals. With our many years of experience, our own research and development capacities and a production site in Neufeld an der Leitha, Austria, we support international companies as a partner for the future.
“With the development, production and distribution of pharmaceutical specialities, Sanochemia has been contributing to people’s health for more than 30 years.”
Our many years of experience and expertise in process development and manufacturing at the highest level make us an important partner to the pharmaceutical industry. Our focus is on products for diagnostics with imaging technology, on sub-areas in urology and neurology as well as on the area of contract manufacturing.
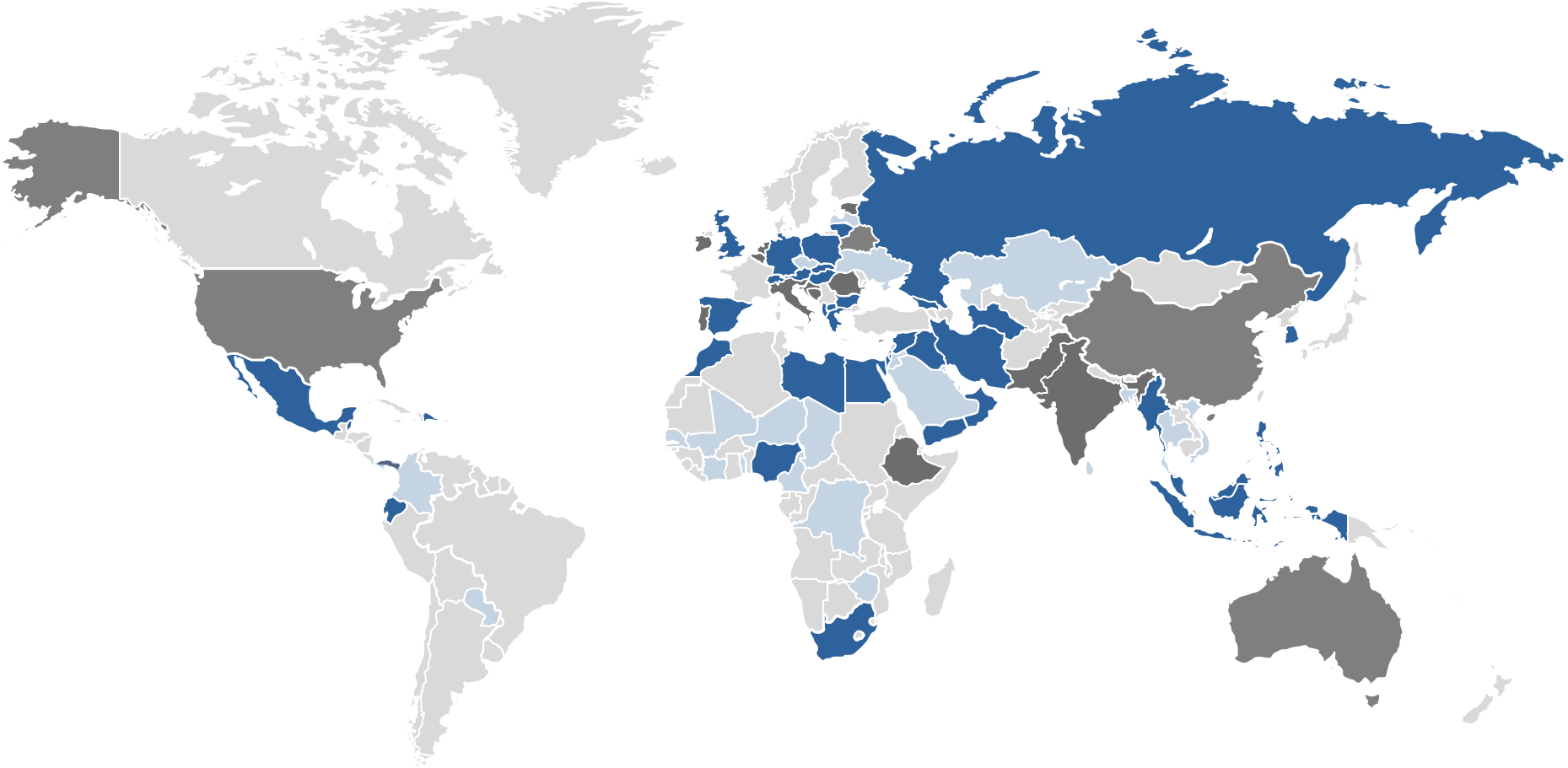
Global presence
We sell products to customers in over 50 countries. On the other hand, further countries on a total of four continents are supplied with sales partners, especially from the regions of the pharmerging markets.
Our Team
Management

Timo Bender

Thomas Erkinger
BUSINESS MISSION
Together for a brighter life
As a renowned Austrian manufacturer of generics and active ingredients, we have special expertise in the field of radiology and, as a contract manufacturer, are a trusted partner of the pharmaceutical industry. Our contrast agents for CT and MR are used worldwide for diagnostic imaging. Our products thus ensure optimal therapeutic decisions for patients.
Throughout the years
1990
Foundation of Sanochemia Pharmazeutika
From Medinger & Söhne and Waldheim Pharmazeutika to Sanochemia Pharmazeutika AG. The company has existed at the site in Neufeld for 130 years. In 1990 the name Sanochemia Pharmazeutika became an important name in the Austrian pharmaceutical industry.
1998
New synthesis facility
An important step into the future was taken with the opening of the new synthesis plant. Galantamine, the active ingredient synthesized exclusively by Sanochemia, is now used in Alzheimer’s therapy.
1999
IPO Frankfurt
The company is listed on the Frankfurt Stock Exchange. A listing on the Third Market of the Vienna Stock Exchange follows in 2010.
2000
Entry into radiology
With Scanlux®/Unilux® an important key product of today’s imaging division joins the company and proves to be a pioneer in the field of international market development.
2001
Expansion of the API facilities
Due to the success of the Synthesis Business Unit, the plant capacity is expanded to 2,000 liters.
2006
Market expansion
Further important milestones in Sanochemia’s development activities are achieved with the products tolperisone hydrochloride, PVP-hypericin and MR-Lux. The MRT contrast agent opens up new opportunities for market expansion.
2012
Opening US market
High-purity tolperisone hydrochloride was licensed in the USA this year from Sanochemia’s own development pipeline. The product is a muscle relaxant which has been further developed by Sanochemia.
2015
Product launch Cyclolux
With the approval of Cyclolux®, Sanochemia has succeeded in bringing the most important product from its own pipeline to market maturity. This covers a particularly low side-effect branch of macrocyclic MRI contrast agents.
2020
Sanochemia Pharmazeutika GmbH
The takeover by a consortium of companies ends the stock market listing and a new chapter begins for a strong pharmaceutical niche supplier in the heart of Europe.
2021
New business areas
The GMP Services business area is launched and new markets are opened up as part of a registration initiative. Sanochemia Labor is founded to perform Covid-19 PCR tests.
Organization
Since April 2020, SANOCHEMIA has been operating as a limited liability company and is now owned by a consortium consisting of EOSS Industries Holding (50%) and b.e imaging (50%).
EOSS Industries is an Austrian powerhouse in building and strategically developing companies. With its divisions Life Sciences, Future Commerce as well as Information Technology, the company serves large customers from numerous industries. EOSS combines the advantages of an integrated technology company with the innovative power and flexibility of a strategic holding company. The medium-sized company has its headquarters in Graz and locations in Austria, Germany and Southeastern Europe.
b.e. imaging is part of the bender group, which is a medium-sized family business with around 150 employees in Germany, Austria and Switzerland. The bender group has been active in the fields of radiology and urology for many decades. The shareholders of the parent companies b.e.imaging and röntgen bender are the families Bender and Eckhardt. The two parent companies have shareholdings in several subsidiaries. Together they all form the bender group.

WE TAKE RESPONSIBILITY
Compliance
social – ethical – economic – environmentally compatible
In addition to manufacturing products of the highest quality, Sanochemia is committed to fulfilling its responsibility towards society and the environment through fair, ethical, sustainable and transparent conduct. In addition to complying with all national and international laws and pharmaceutical guidelines, our employees are obliged to act in accordance with Sanochemia’s principles
Regular training sessions are held on the subject of quality compliance to ensure that all employees are familiar with the principles and adhere to them. Managers and employees are expected to act in a socially, ethically and economically responsible manner, conserve resources and protect the environment.
We operate worldwide through a large number of distribution partners. We work together with partners on quality compliance requirements and communicate our principles. We support them in complying with all regulatory requirements as well as in a responsible approach to resources, the environment and employees.
Avoidance: Adherence to policies, training, counseling of employees
Recognize Holistic risk assessment, internal audit program
Response: Clarification of incidents, definition of corrective and preventive measures, intensive cooperation with authorities
Drug Safety
We collect and document the occurrence of side effects with our products.
If you wish to report a side effect, please contact us by phone at +43 507266444. Please send e-mails in addition to this to: drug.safety@sanochemia.com
In the event of a side effect report, please provide us with at least the following data:
- The product concerned
- information on the patient (age, sex)
- Possible detailed description of the side effect
- Your contact details (for possible queries)
INNOVATION FOR THE FUTURE
F&E and Cooperations
The needs of the patients, customers and medical personnel provide SANOCHEMIA with incentive for new developments. The priorities are innovation and further development, as well as the manufacture of active ingredients and liquid to semi-solid forms of dosage. The emphasis rests on products that help expand the portfolio and are manufactured in our own production facilities. These improve the value-added chain in the long term.
In our innovation department, we focus on constantly evaluating new ideas and modern technologies to promote future developments. This means that we seize the opportunity to further develop products and production processes, making them more efficient while minimizing our environmental footprint. Our development collaborations help to gain new perspectives and are an important pillar for Sanochemia’s future.
Fundamental research and the assessment of research concepts are covered by a network of partnerships with academic research institutions and biotech companies. Specialist knowledge in developmental and regulatory strategies was built up over many years. SANOCHEMIA performs new authorisation procedures in the field of pharmaceutical regulation every year, and maintains global authorisations as well. Numerous global patents and patent applications protect the company’s research and development ideas.
LICENSE PARTNER

Exclusive license agreement for the development and commercialization of tolperisone in the USA.
COOPERATION PARTNERS

Research collaboration on the effect of anti-tumor peptides in malignant melanoma.
SUSTAINABILITY IS IMPORTANT TO US
Sanochemia environment and energy program
The goal of Sanochemia’s sustainability program is to produce in a more energy-efficient and resource-friendly manner. For us, this also includes the subject of innovation under the aspect of developing “green” processes. The basis for success is the creation of awareness among all employees and partner companies. Together, we are constantly improving the company’s sustainability footprint. The following cornerstones form the basis for our contribution to a better future:
We produce energy
With recovered combustion energy from the synthesis we supply the Viennese with heat in their households. Furthermore, since 2023, we have an in-house photovoltaic facility that produces more than IOO% of the electricity required for our Plant 2 in Neufeld.
We plant trees
Our extensive property in Neufeld an der Leitha provides the best conditions for making a visible contribution to the environment. We use our large acreage to plant new trees, preserve old growth, and create habitat for native insects and bees.
We take care of resources
We are constantly optimizing our production and developing new processes to improve resource consumption: whether through the modernization of production facilities, optimization of night shutdowns, development projects for “green” active ingredient synthesis, investments in e-mobility or the digitalization of processes. In the fourth quarter of 2022, our efforts reduced gas consumption by 33%.
We stand for cooperation
Social responsibility, taking care of each other and working together for a better future. We stand for an open, appreciative and results-oriented work culture that always keeps environmental and sustainability aspects in mind.